Introduction:
Carbon, the fourth-most abundant element in the universe, is a remarkable building block of life and an essential component of countless substances. From diamonds to DNA, carbon’s versatility knows no bounds. In this extensive exploration, we will delve into the multifaceted world of carbon, its diverse forms, applications across various industries, and its crucial role in shaping the natural world and technological advancements.
The Basics of Carbon:
Carbon, with the symbol ‘C’ and atomic number 6, belongs to Group 14 of the periodic table. Its unique ability to form strong covalent bonds with other carbon atoms enables the creation of a vast array of compounds. Carbon exhibits various allotropes, the most common of which are diamond, graphite, and amorphous carbon.
Allotropes of Carbon:
1. Diamond:

Structure:
Each carbon atom forms four strong covalent bonds in a tetrahedral arrangement, creating a three-dimensional crystal lattice.
Properties:
Known for its exceptional hardness, high refractive index, and excellent thermal conductivity.
Applications:
Besides being a precious gemstone, industrial diamonds are crucial for cutting, grinding, and drilling due to their hardness.
2. Graphite:

Structure:
Carbon atoms arrange in hexagonal rings, forming flat, parallel layers held together by weak van der Waals forces.
Properties:
Exhibits lubricating properties, electrical conductivity, and is relatively soft.
Applications:
Widely used as a lubricant, in pencils, and as a moderator in nuclear reactors.
3. Fullerenes:
Structure:
Hollow spheres, ellipsoids, or tubes composed of carbon atoms.
Properties:
Unique cage-like structures with potential applications in medicine, electronics, and materials science.
Applications:
Research focuses on drug delivery, superconductors, and reinforcement materials.
4. Carbon Nanotubes:
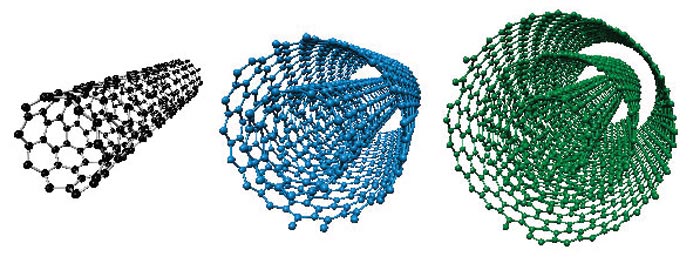
Structure:
Cylindrical tubes with remarkable mechanical, electrical, and thermal properties.
Properties:
High tensile strength, excellent conductors of electricity, and efficient heat conductors.
Applications:
In nanotechnology, used in electronics, materials science, and even potential applications in space elevators.
5. Graphene:

Structure:
A single layer of carbon atoms arranged in a hexagonal lattice.
Properties:
Extraordinary strength, excellent conductor of electricity, and transparency.
Applications:
In electronic devices, flexible electronics, energy storage, and various materials.
Carbon in the Natural World:

1. Organic Chemistry:Carbon is the backbone of organic molecules, forming the basis of life. All living organisms, including humans, consist of carbon-based compounds such as proteins, carbohydrates, lipids, and nucleic acids.
2. Carbon Cycle:The carbon cycle is a crucial ecological process involving the exchange of carbon among the atmosphere, oceans, soil, rocks, and living organisms. Photosynthesis and respiration play pivotal roles in this cycle.
3. Fossil Fuels:Coal, oil, and natural gas are fossil fuels primarily composed of carbon. Combustion of these fuels’ releases carbon dioxide, contributing to the greenhouse effect and climate change.
4. Carbon Sequestration:Forests and oceans act as carbon sinks, absorbing and storing carbon dioxide. Deforestation and pollution threaten these natural mechanisms, emphasizing the importance of conservation efforts.
Carbon in Technology and Industry:
1. Carbon Composites:
The aerospace and automotive industries extensively use carbon composites for their exceptional strength-to-weight ratio. Carbon fibber-reinforced polymers are vital in manufacturing lightweight, high-performance components.
2. Carbon in Electronics:
Semiconductors, an essential component of electronic devices, often utilize carbon-based materials. Graphene, with its remarkable electronic properties, holds promise for future electronic applications.
3. Energy Storage:
Carbon materials, including graphite in lithium-ion batteries, play a pivotal role in energy storage technologies. Ongoing research explores the potential of carbon-based materials for advanced batteries and supercapacitors.
4. Water and Air Purification:
Activated carbon is widely employed for water and air purification due to its high surface area and adsorption capabilities. It effectively removes impurities, contaminants, and pollutants.
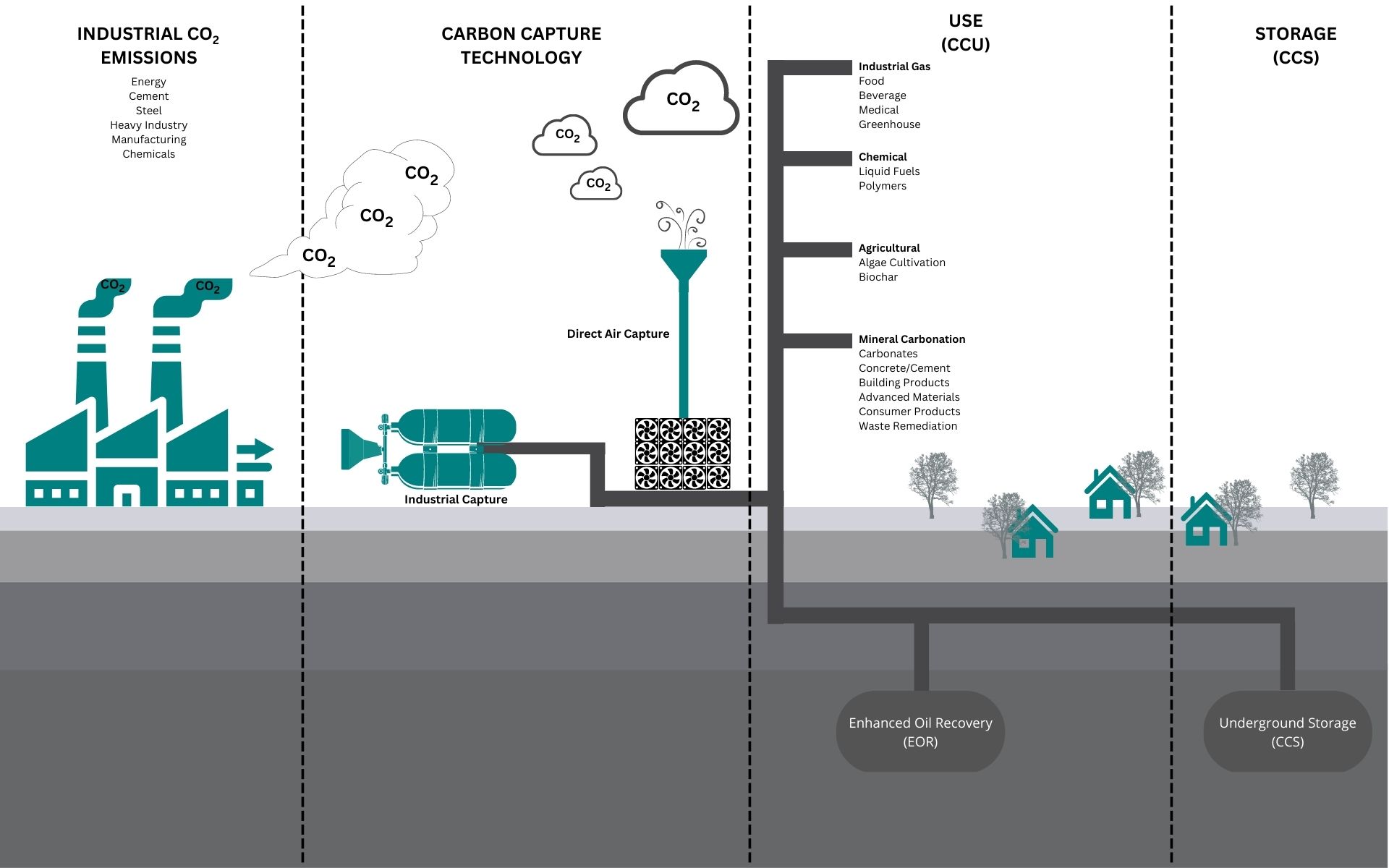
5. Carbon Capture and Storage (CCS):
CCS technologies aim to capture carbon dioxide emissions from industrial processes and power plants, preventing them from entering the atmosphere. These captured emissions are then transported and stored underground.
Environmental Impact and Concerns:
1. Climate Change:
The burning of fossil fuels releases carbon dioxide, contributing to global warming and climate change. Sustainable practices and a shift toward renewable energy sources are essential for mitigating these impacts.
2. Deforestation:
Deforestation disrupts the carbon cycle, releasing stored carbon into the atmosphere. Reforestation efforts and sustainable forestry practices are crucial for maintaining ecological balance.
3. Ocean Acidification:
Increased carbon dioxide levels in the atmosphere lead to higher absorption by the oceans, causing acidification. This poses a threat to marine ecosystems, particularly coral reefs and shell-forming organisms.
Also read this “Trails Carolina Death List: An In-Depth Examination of Allegations “
Conclusion:
Carbon, with its diverse forms and unparalleled versatility, is integral to life, industry, and technological advancements. From the intricate structures of diamonds to the potential breakthroughs promised by graphene, carbon continues to captivate researchers, engineers, and environmentalists alike. Understanding and harnessing the power of carbon is key to addressing environmental challenges, advancing technology, and ensuring a sustainable future. As we navigate the intricate web of carbon’s applications, both beneficial and concerning, the need for responsible stewardship of this remarkable element becomes increasingly evident.
FAQs:
1. Is carbon present in all living organisms?
Yes, carbon is the fundamental building block of organic molecules, and it is present in all living organisms.
2. How does carbon contribute to climate change?
The combustion of fossil fuels releases carbon dioxide into the atmosphere, contributing to the greenhouse effect and climate change.
3. What are some everyday products that contain carbon-based materials?
Everyday products such as plastics, textiles, medicines, and electronic devices often contain carbon-based materials.
4. Can carbon capture and storage effectively reduce carbon emissions?
Carbon capture and storage (CCS) technologies have the potential to reduce carbon emissions from industrial processes and power plants, contributing to climate change mitigation.
5. What is the significance of carbon in nanotechnology?
Carbon-based nanomaterials, including graphene and carbon nanotubes, have unique properties that make them valuable in various nanotechnological applications, from electronics to medicine.